
Fig. 1
Indiana's best-known and most abundant mineral is
Calcite forms in a huge (more than 12,000) number of shapes. It has a rhombohedral structure (fig. 2) and frequently shows hexagonal crystals. Calcite is relatively soft with a scratch hardness of three on the Mohs scale. It shows excellent rhombohedral cleavage (fig. 3). Clear crystals demonstrate double refraction (fig. 4) and other optical properties. Crystals can grow quite large with some several feet in size.
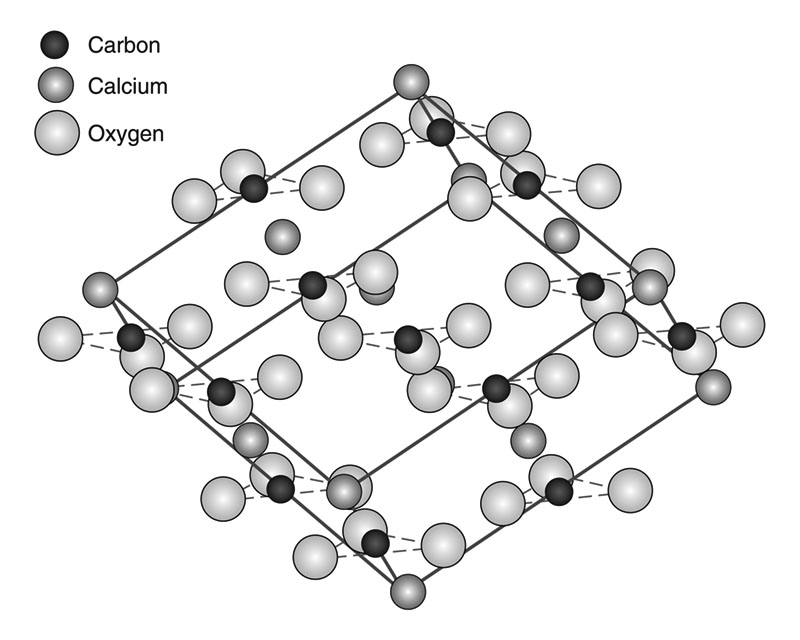
Fig. 2) Rhombohedral model
Calcite is present in many forms here in Indiana. The most common is as tiny crystals or fossil fragments that comprise limestone. Calcite can form by direct precipitation from waters rich in calcium. As concentrations increase or the amount of water decreases crystals of solid calcite crystals form. Organisms make structural elements or shells. Many Indiana rock units are made mostly of calcite (fig. 5) crystals or fossil fragments. These pieces occur in various sizes, shapes, and intergrowths. Detailed studies describe and differentiate many varieties of the most useful limestone.
Limestone is a major raw material for Indiana and most developed communities. Limestone is mined at 96 sites in Indiana (see industrial minerals) most is used to make aggregate for concrete, roads, buildings, and such. Limestone has many other uses. Indiana limestone is used for making glass and a myriad of other products. Pure limestone (more than 90 percent calcite) command an excellent price as chemical raw materials.

Fig. 3) Cleavage
Mineral collector's value calcite crystals and Indiana hosts some excellent crystals. A rock collector's journal featured a large, showy calcite crystal on its issue devoted to minerals and fossils of Indiana. Most crystals are clear, white, amber, or brown. Excellent crystals of Indiana calcite can be found in museums all over the United States. Calcite is also abundant in Indiana's geodes. Perfect crystals of many shapes and colors are found in many geodes. Many geodes are completely filled with calcite. Some calcite even glows in fluorescent light.
Calcite is closely related to aragonite (also CaCO3) which is a polymorph. Calcite is also related to dolomite [CaMg(CO3)2]. These and distant cousins siderite (FeCO3), strontianite (SrCO3), and ankerite [CaFe(CO3)2] make up a group of minerals known as carbonate minerals. The group makes a vital and intriguing part of Indiana's natural heritage.
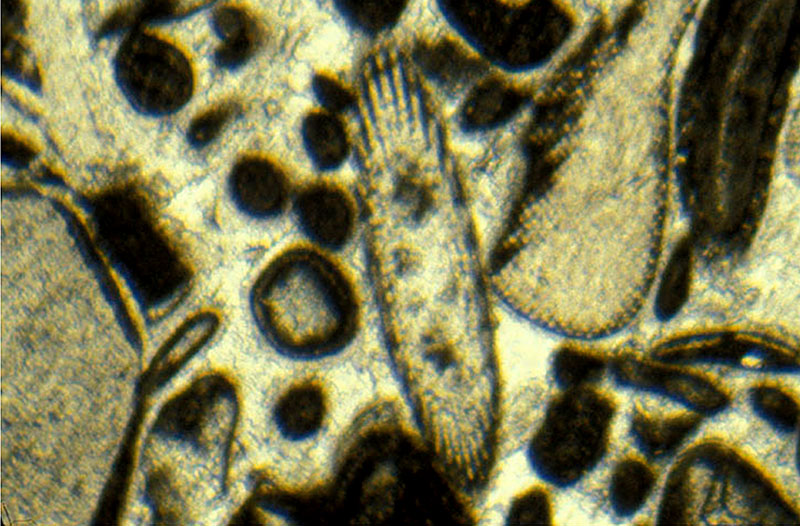
Fig. 5) Calcite crystal thin section & fossil

Fig. 4) Double refraction


